Ocean acidification threatens shellfish in estuaries, coastal ecosystems

Whitman Miller of the Smithsonian Environmental Research Center checks an experimental aquarium used to rear juvenile oysters. (Smithsonian Environmental Research Center)
MECHANICSVILLE, Va. — The shellfish of the Chesapeake Bay and other estuaries in the Mid-Atlantic region face a host of threats to their survival. Overfishing, pollution, sedimentation, habitat destruction, and introduced species have taken a toll on many shelled species, such as the eastern oyster (Crassostrea virginica), that play important roles in maintaining the health of the estuarine ecosystems. A new study, led by Whitman Miller (Smithsonian Environmental Research Center, Edgewater, Maryland, USA), finds another potentially disastrous threat coming from the atmosphere — ocean acidification.
The findings have significant implications for efforts to restore the Chesapeake Bay ecosystem.
“Our results suggest that the native eastern oyster is quite sensitive to elevated CO2/reduced pH,” Miller said. “Future restoration efforts should probably consider the chemical conditions of waters when selecting locations for oyster restoration.”
Ocean Acidification Links:
A. Whitman Miller, Amanda C. Reynolds, Cristina Sobrino, and Gerhardt F. Riedel. 2009. Shellfish Face Uncertain Future in High CO2 World: Influence of Acidification on Oyster Larvae Calcification and Growth in Estuaries. PLoS ONE 4(5): e5661. doi:10.1371/journal.pone.0005661
NOAA/PMEL Ocean Acidification Home Page
European Project on Ocean Acidification
Marine Climate Change Impacts Partnership — Acidification
The Ocean Acidification Network
The acidification of the oceans is driven by carbon dioxide released into the atmosphere as a result of our combustion of fossil fuels. While some debate whether or not humans are causing climate change by these carbon dioxide emissions, the effect on the oceans is clear. Carbon dioxide in the air readily dissolves in water, making the water more acidic by the formation of carbonic acid.
Acidity is measured by pH — a scale from 0 to 14 in which lower numbers mean more acidic conditions and higher numbers mean more basic (alkaline) conditions. Neutral pH (neither acid nor alkaline) is 7. The scale is logarithmic, such that a decrease of 1 unit on the scale — from 7 to 6, for example, represents a tenfold increase in acidity level.
Since the beginning of the Industrial Revolution, the pH of the oceans has dropped by 0.1 — while the number seems small, it actually represents a 30 percent increase in acidity.
The primary concern is how the increased acidity affects shellfish species that build their shells from carbonate minerals — primarily aragonite and calcite — extracted from the water. Acidic waters easily dissolve these minerals, making it harder for the animals to extract them from the water and in turn build shells. [Remember what happened to the baking soda (sodium bicarbonate) after the addition of vinegar (acetic acid) in the elementary school volcano experiment?]
So far, most of the concern over ocean acidification was focused on marine ecosystems such as coral reefs which are built from carbonate minerals deposited by reef-building corals and algae. Given the differences in physical conditions between oceanic and estuarine environments, the new study, published last Wednesday in the journal PLoS ONE, assessed whether shellfish in estuaries and coastal environments would be more vulnerable than those in oceans.
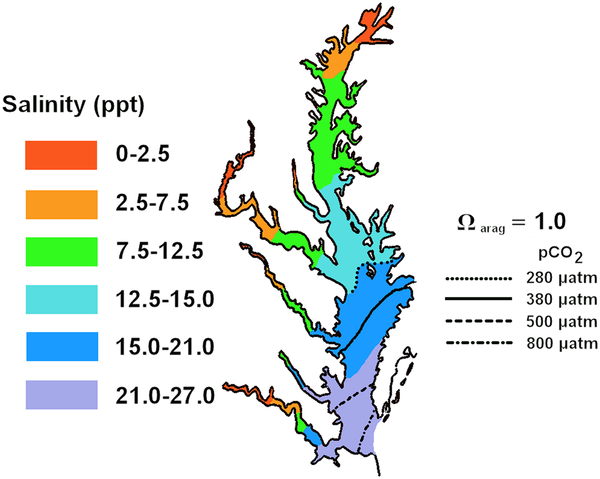
Projected mean summer positions of aragonite compensation points for the Chesapeake Bay under preindustrial, present, and predicted atmospheric CO2 levels. (From Miller et al., 2009)
Estuaries and nearshore coastal ecosystems are less deep, less saline, and less alkaline than the open ocean. Any one of the three conditions reduces the ability to buffer against changes in pH. The compensation point — the point below which it becomes more expensive in terms of energy required for organisms to extract carbonate minerals from the water — is also a function of the salinity and temperature of the water. Lower pH, lower salinity, and increasing temperatures all increase the compensation point, shifting it seaward, i.e., reducing the area where conditions are favorable for shell production.
Given those conditions, Miller and his colleagues suspected that larval oysters would be the most severely affected as they relied more on aragonite — the more soluble of the two minerals — to form their shells while mature oysters relied more on calcite.
To test its hypothesis, the research team grew larval oysters of two species, eastern oyster and Suminoe oyster (Crassostrea ariakensis), a species native to Asia, in estuarine water under four different atmospheric concentrations of carbon dioxide — 280, 380, 560, and 800 ppm. The concentrations correspond with preindustrial, current, and projected atmospheric concentrations of atmospheric carbon dioxide 50 and 100 years from now.
Suminoe oysters showed no noticeable effect from the increased carbon dioxide, either in terms of growth (shell area) or in terms of shell calcium content. Eastern oysters, however, showed a 16 percent decrease in growth rate and a 42 percent decrease in calcium content when comparing preindustrial to year 2100 carbon dioxide concentrations.
Miller and colleagues conclude that, while the effects of acidification will vary from species to species and place to place, it may lead to reductions in growth or even in the geographic distribution of estuarine shellfish. Given the energy costs of shell formation, the increasing difficulty in extracting carbonate minerals from the water may force the animals to take energy away from other important processes, such as immune defense or reproduction. This may be exacerbated by other stressors in the environment, such as extreme temperatures and pollution. Slower shell formation and growth may — by forcing larvae to spend longer periods in the planktonic, or floating, stage — reduce the percentage of oyster larvae surviving to maturity.
The researchers are very concerned about the future of estuarine and coastal ecosystems, which because of their greater environmental variability, can be much more stressful for their inhabitants than open ocean ecosystems. Disastrous environmental and economic consequences may result if organisms that form shells based on carbonate minerals give way to those that do not.
Chris French, Virginia Director of the Alliance for the Chesapeake Bay, would like to see this research confirmed by other studies as well as expanded to other mollusk species, such as clams. He also wonders what the consequences may be for those who make their living on the water.
“It’s definitely concerns me that, given the current state of the oyster population, it looks like we have another potential challenge ahead of us as far as the restoration of the native oyster,” French said. “It definitely raises some additional concerns, not just on the ecology of the Bay restoration effort, but also of the economic implications because we’ve seen so many people in the watermen’s sector whose livelihoods depend on the shellfish resources.”
— David M. Lawrence
Leave a Response
You must be logged in to post a comment.



You must be logged in to post a comment.